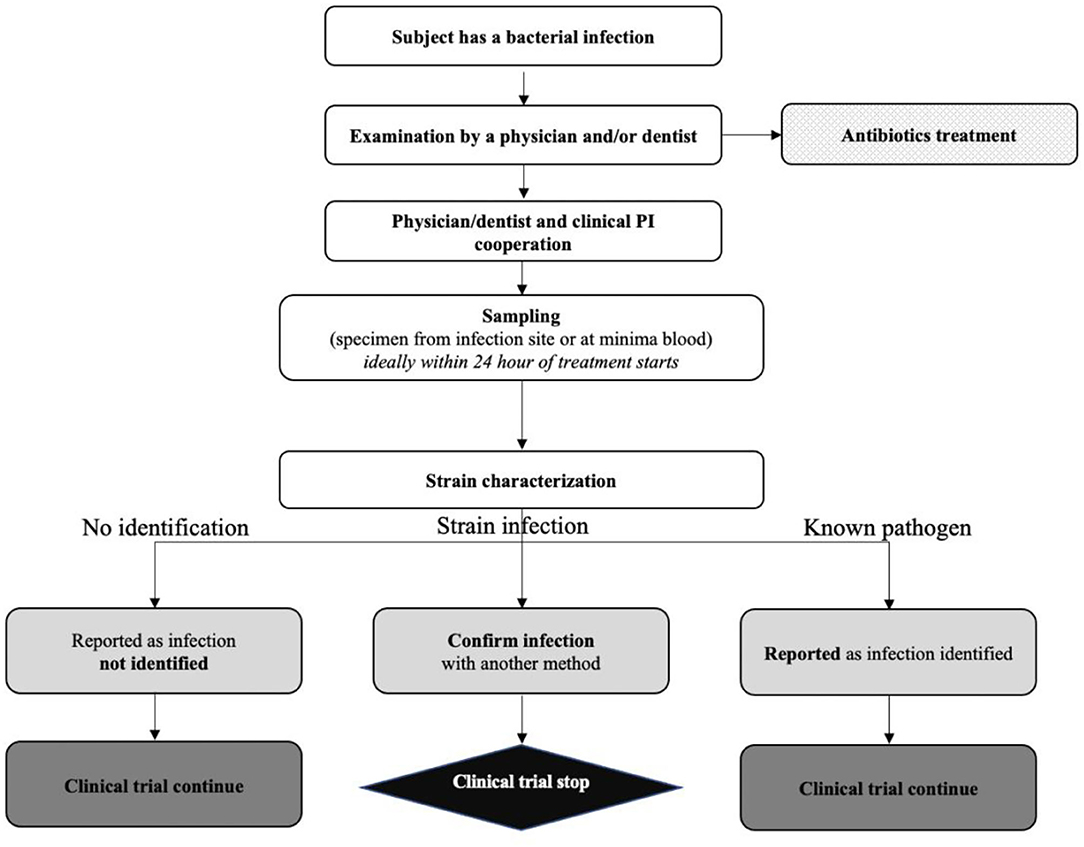
Frontiers | Entering First-in-Human Clinical Study With a Single-Strain Live Biotherapeutic Product: Input and Feedback Gained From the EMA and the FDA

Effect of body size and posture on limb EMA. (A) Hindlimb EMA scaling... | Download Scientific Diagram

CONSORT flow chart of the prospective cohort study. EMA ¼ endovenous... | Download Scientific Diagram

Janssen seeks EMA approval for single tablet pulmonary arterial hypertension treatment - Pharmaceutical Technology

Comparison of regulatory pathways for the approval of advanced therapies in the European Union and the United States - Cytotherapy

EU Regulatory Pathways for ATMPs: Standard, Accelerated and Adaptive Pathways to Marketing Authorisation: Molecular Therapy Methods & Clinical Development

Necessity of strengthening the current clinical regulatory for companion diagnostics: An institutional comparison of the FDA, EMA, and MFDS: Molecular Therapy Methods & Clinical Development

EU Regulatory Pathways for ATMPs: Standard, Accelerated and Adaptive Pathways to Marketing Authorisation - ScienceDirect
CONSORT flow chart of the prospective cohort study. EMA ¼ endovenous... | Download Scientific Diagram

One step closer: digital readouts of walking as a measure of health | IMI Innovative Medicines Initiative

MRI From Your Smartphone? Auto Billing When You Step Out of the Exam Room? Yes! // New HSS Study to Tackle Critical ACL, UCL Failure Issue | Orthopedics This Week
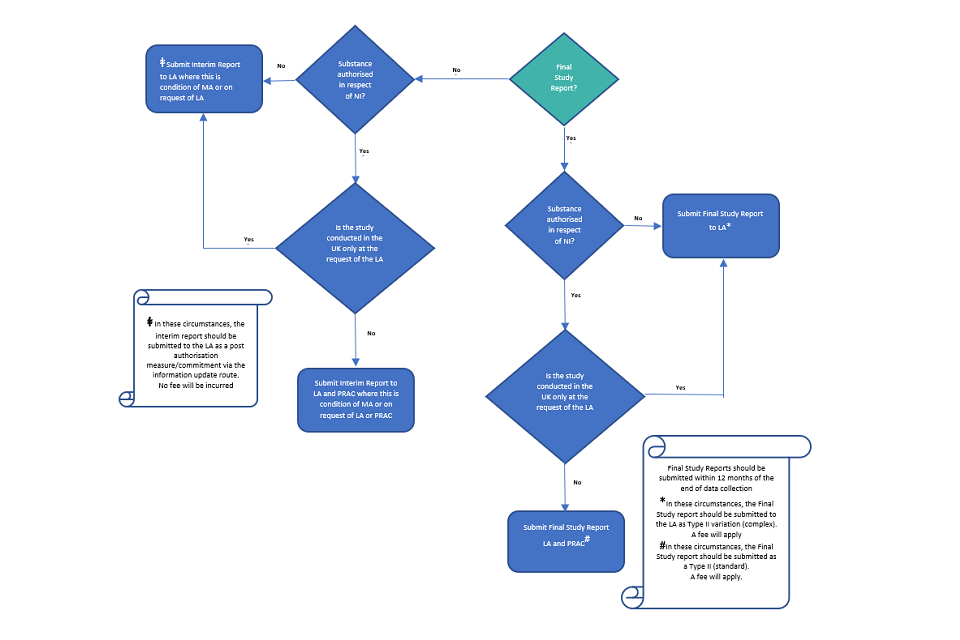
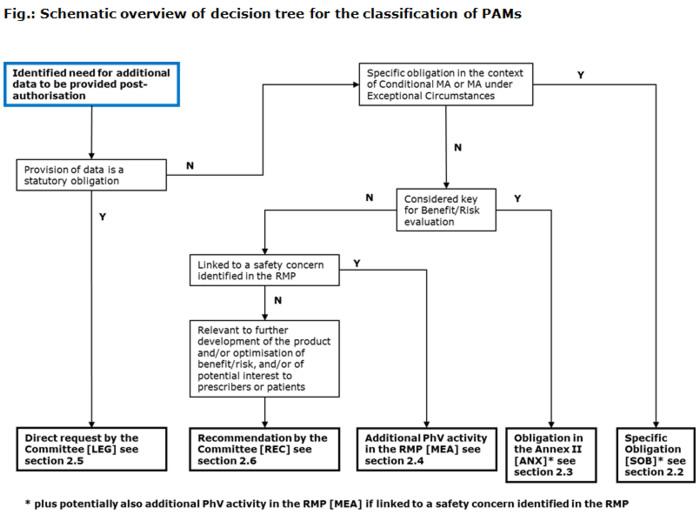
European Medicines Agency post-authorisation procedural advice for users of the centralised procedure

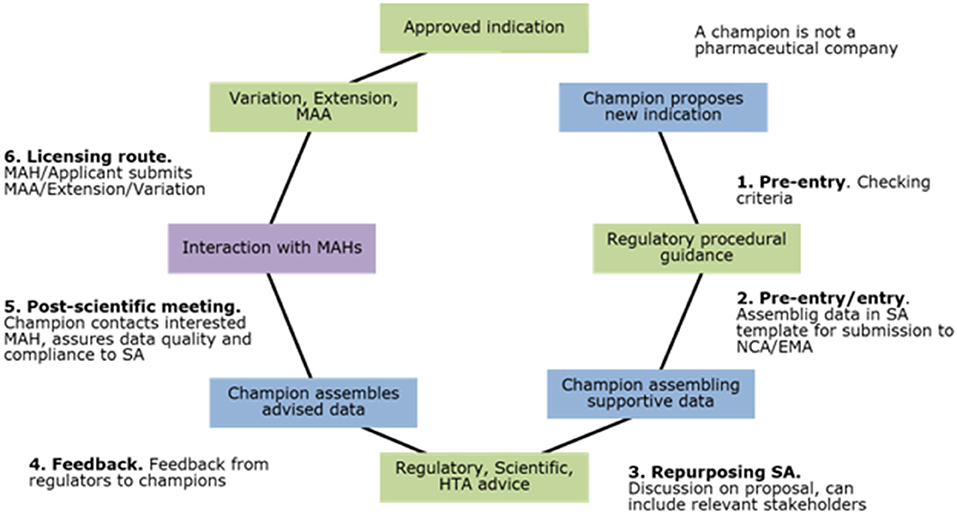
Frontiers | A Regulatory Risk-Based Approach to ATMP/CGT Development: Integrating Scientific Challenges With Current Regulatory Expectations