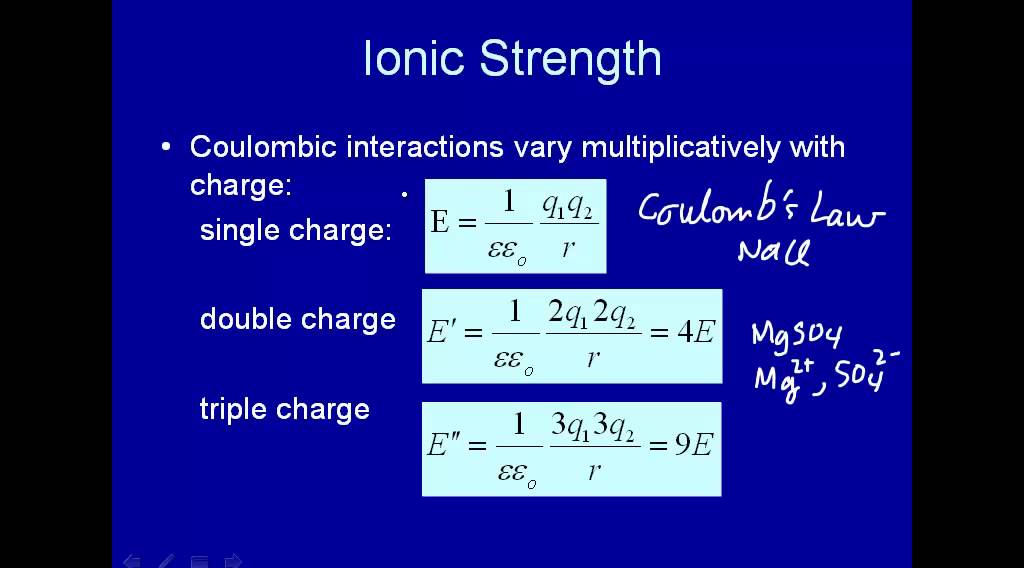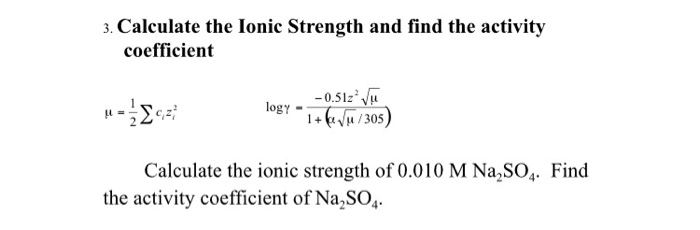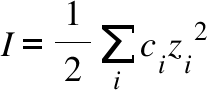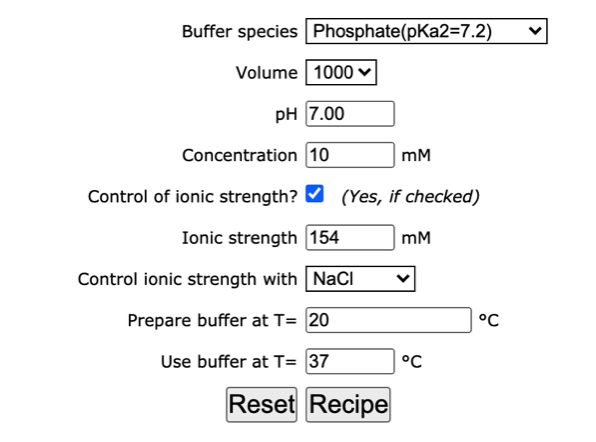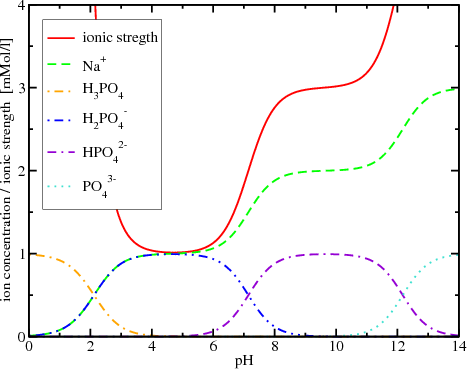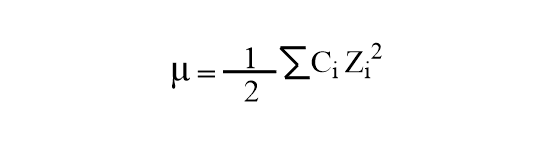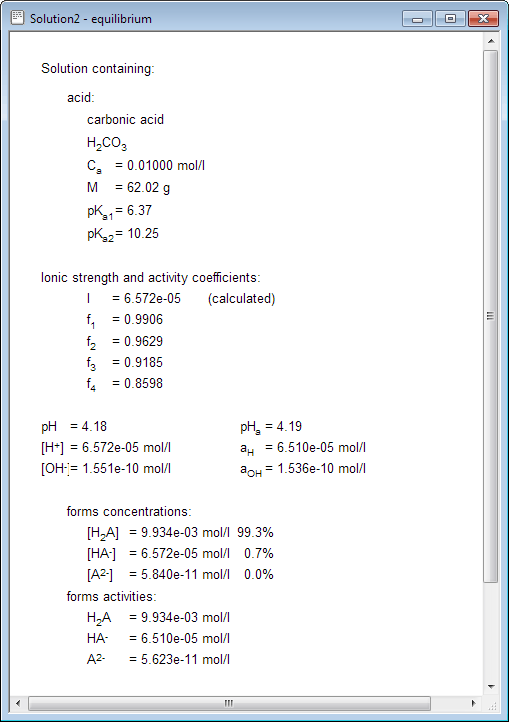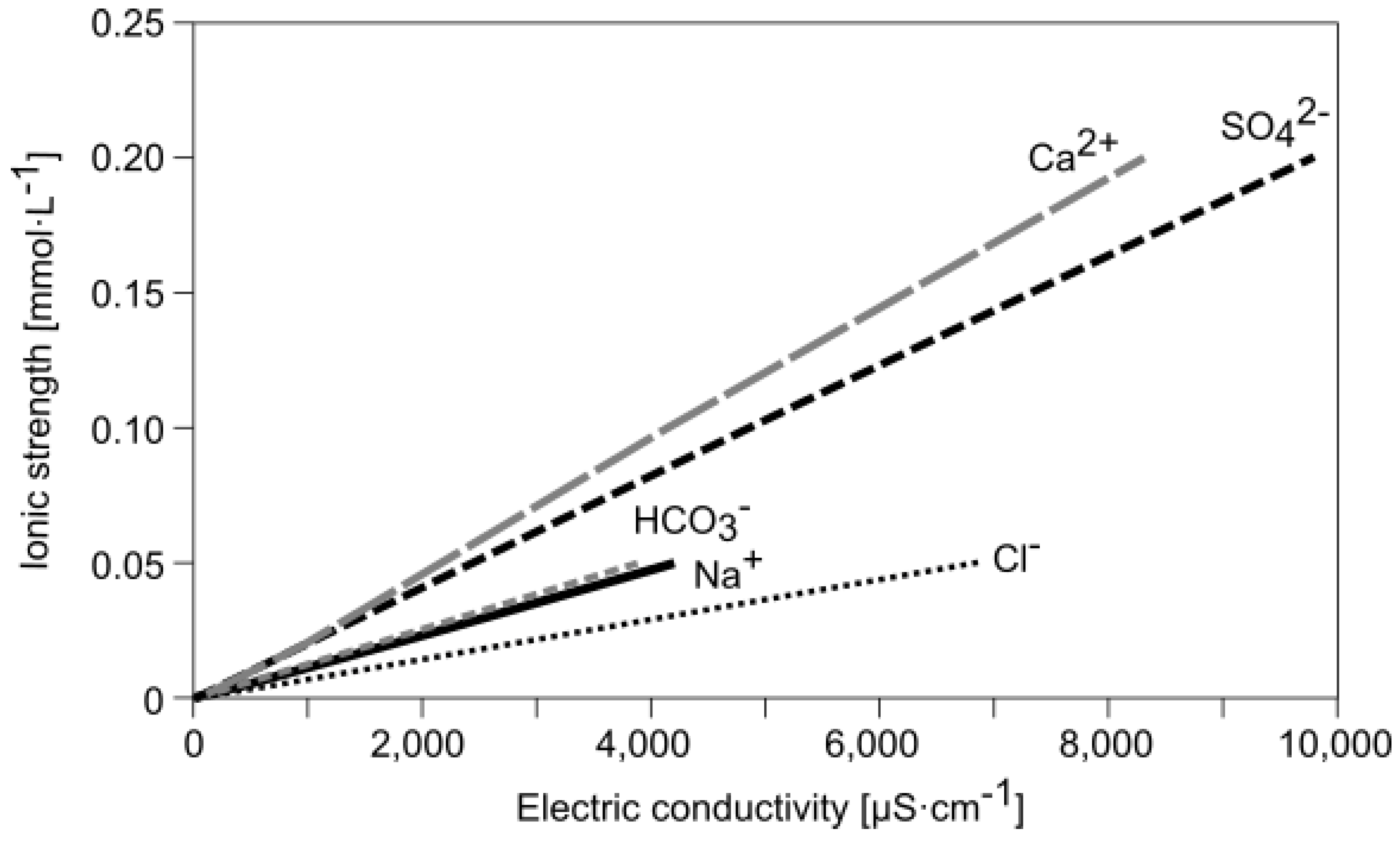
Water | Free Full-Text | Empirical Formula to Calculate Ionic Strength of Limnetic and Oligohaline Water on the Basis of Electric Conductivity: Implications for Limnological Monitoring
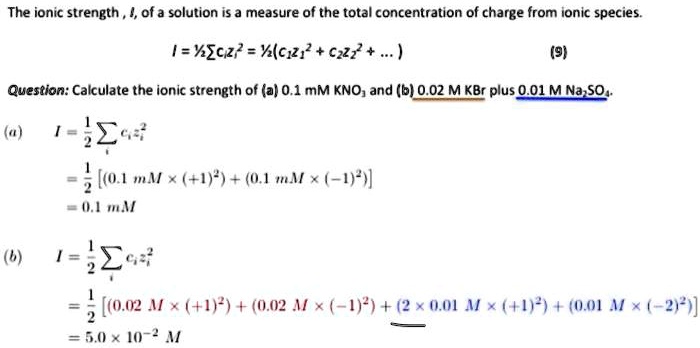
SOLVED: The ionic strength of a solution is a measure of the total concentration of charge from ionic species. (a) Calculate the ionic strength of 0.1 mM KNO3: I = (0.1 mM * (+
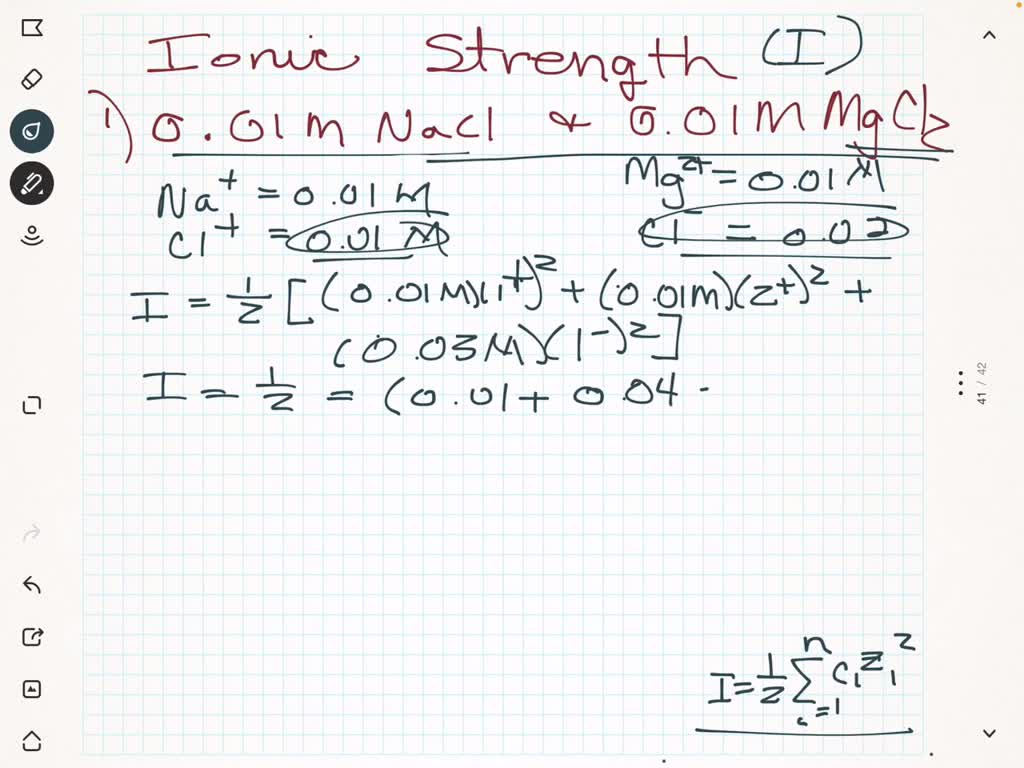
SOLVED: Which solution below has the highest ionic strength? 0.01 M NaCl and 0.01 M MgCl2 0.02 M NaCl 0.02 M MgCl2 They - will all have the same ionic strength:

physical chemistry - Calculating the ionic strength of a histidine solution - Chemistry Stack Exchange